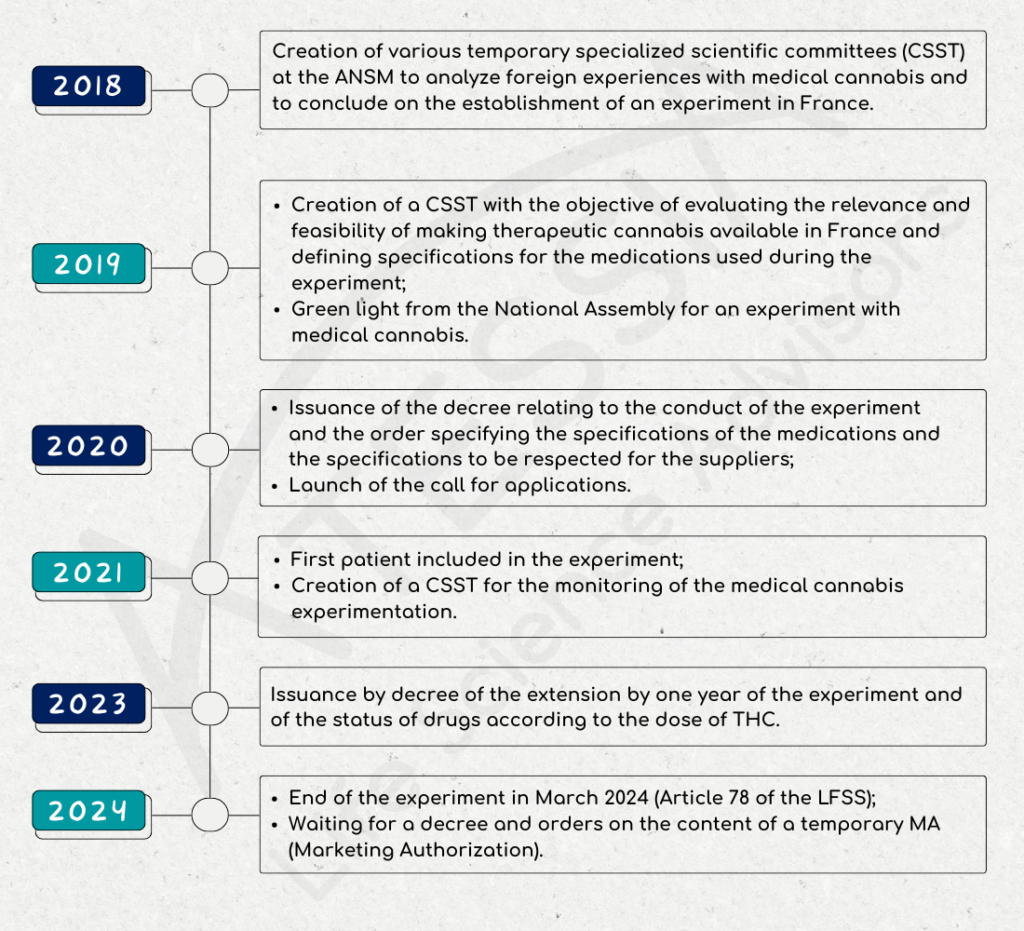
The Excellence of ATESSIA’s CMC: Your Partner in Technical and Regulatory Affairs
In a constantly evolving pharmaceutical industrial environment, regulatory requirements for quality data are becoming increasingly complex. Health authorities are continually updating guidelines, increasing CMC demands on regulatory affairs professionals. Faced with this complexity, revising the operational model and utilizing external expertise are essential solutions to overcome these challenges and meet market demands.
Why Outsource CMC Activities?
- Increasing Regulatory Requirements Health authorities are continuously raising the quality standards from development to drug registration and throughout the lifecycle of marketing authorizations (MA**). This trend imposes a significant workload from a CMC perspective, necessitating rigorous and expert management of regulatory dossiers.
- Impact of Mergers and Acquisitions Corporate mergers and acquisitions increase the number of regulatory submissions to reflect changes in supply sources, production site transfers, etc. This dynamic requires increased flexibility and responsiveness to maintain compliance and the continuity of pharmaceutical operations.
- Resource Management Optimization Increasingly complex regulatory requirements necessitate deep knowledge, leading to increased personnel needs and creating a talent shortage. The traditional operational model of pharmaceutical laboratories is often no longer optimized to handle the surplus work related to these CMC activities. Outsourcing complex technical and regulatory aspects reduces workload and improves overall efficiency.
ATESSIA’s Unique Expertise
ATESSIA was founded by Géraldine Baudot-Visser, a recognized expert in the technical-regulatory field. With a doctorate in pharmacy, extensive experience in R&D and regulatory affairs, Géraldine created ATESSIA to offer an innovative and client-centered approach. Her solid CMC expertise, acquired within major pharmaceutical laboratories and consulting firms, is at the heart of the service offering.
Why Choose ATESSIA for Your CMC Needs?
- Cutting-Edge Expertise and Knowledge ATESSIA has multidisciplinary expert teams with in-depth CMC knowledge. Our consultants possess practical experience and a fine understanding of regulatory authorities’ expectations, ensuring the quality of dossiers and compliance with current requirements.
- Cost Reduction and Time Savings ATESSIA has the expertise to efficiently manage CMC activities and offers economical and fast solutions, best adapting to the market entry deadlines desired by its clients.
- Guaranteed Quality Structured around the ISO 9001 standard, ATESSIA ensures impeccable quality at every stage of the product lifecycle.
- Partner for Your R&D Activities ATESSIA has Research Tax Credit approval, enabling you to be supported in your research and development activities.
Our Commitment to Excellence and Innovation
At ATESSIA, we stand out with our agile and tailored approach, integrating feedback and specific needs of each client. Our flexibility and ability to integrate new technologies allow us to offer innovative solutions adapted to evolving market demands.
Choosing ATESSIA for your CMC needs ensures expert and personalized support, capable of transforming your regulatory challenges into successes. Our commitment to excellence and innovation guarantees optimal results that ensure your competitiveness in the market.
To learn more about our CMC services and other regulatory and pharmaceutical affairs offerings, and to discover how we can support you, contact us today.
hello@atessia.fr
*CMC: Chemistry Manufacturing and Control
**MA: Marketing Authorization