Following the “Médiator” case and inspired by the Sunshine Act in the United States, the law of December 29, 2011, concerning the strengthening of health safety, known as the “Bertrand Law,” was passed.
This law establishes systematic transparency of links between health industries on one hand, and other actors in the health field on the other. This primarily includes healthcare professionals, but also students, learned societies, associations, media, etc.
The “transparency of links” system, which provides the general public with information on the relationships between health industries and members of the health system, is in line with the “regulation of benefits” system, which aims at the ethics of healthcare professionals. These two legislations interact with each other, with some subtle specificities that distinguish them.
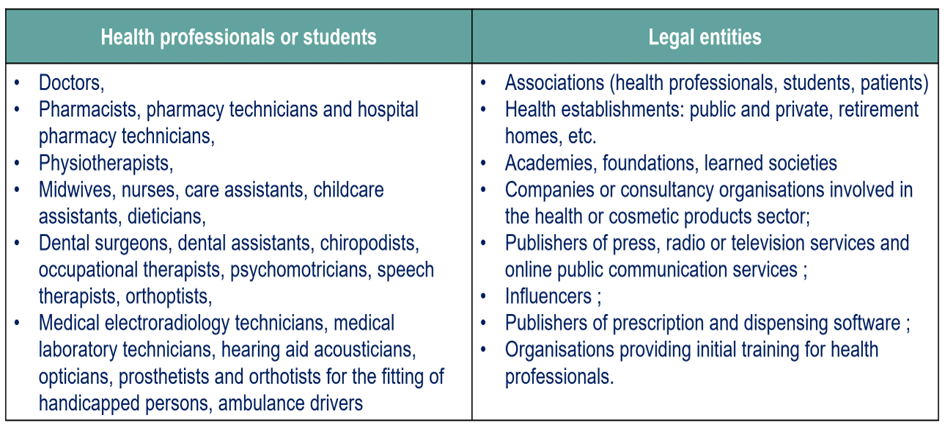
Who is Affected by the System?
The following actors are concerned:
Types of Declaration and Publication
It is appropriate to publicly disclose retrospectively:
- Agreements: publication of all contracts made between laboratories and a health actor (for example: agreements with coverage of hospitality expenses (catering, transport, accommodation, registration fees at a congress));
- Service provision contracts (for example: remuneration for clinical expert work);
- Benefits exceeding 10 euros including taxes (for example, the granting of health product samples);
- Remunerations awarded (for example: remuneration of a healthcare professional for speaking at an event).
Commercial contracts are excluded.
The typology of benefits and agreements responds to precise definitions that have been clarified by the authorities.
The system requires that pharmaceutical companies, in particular, are obliged to publish information on the public database https://www.transparence.sante.gouv.fr, according to the following frequency:
- Publication twice a year:
- First calendar semester, i.e. from January 1st of year N to June 30th of year N: submission on the “transparency-health” website by September 1st of year N.
- Second half of the calendar year, i.e. from 1 July of year N to 31 December of year N: submission on the “transparency in health” website no later than 1 March of year N+1.
Thus, the general public can access the work links and professional relationships maintained between health industries and actors in the French healthcare system.
In parallel, the European Federation of Pharmaceutical Industries and Associations (EFPIA) has also adopted the “EFPIA code of practice” to frame collaboration with Healthcare Professionals or their Organizations when conflicts of interest exist. This text is applicable to member companies.
ATESSIA supports its clients on all these questions.
Article written by Zarine RAMJAUNY, Junior Legal Consultant