Atessia assists its clients on a daily basis with the practicalities of implementing the French early access programs and compassionate use scheme, the subtleties of which require some explanation.
This new system has been in place since 1st July 2021 and is based on 2 access and reimbursement mechanisms:
- Early access (AAP)
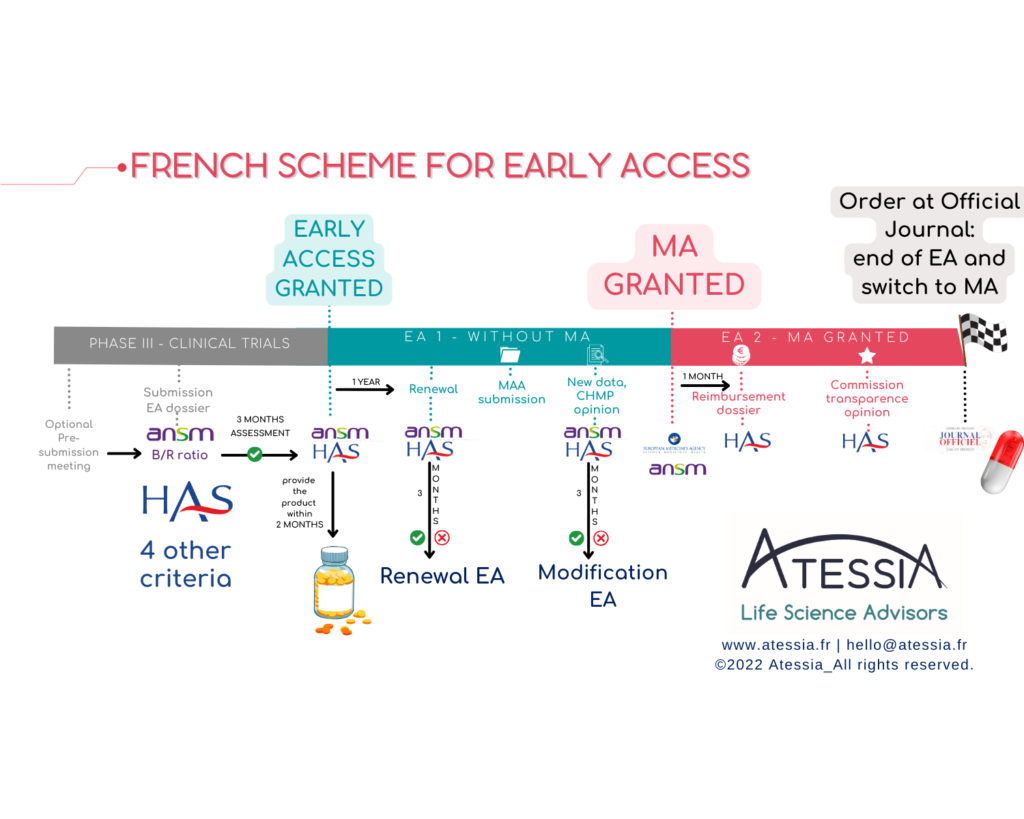
The first is the early access scheme, dedicated to medicinal products that meet an unmet therapeutic need and are likely to be innovative. The pharmaceutical company submits an application for early access authorisation (AAP) to the French National Authority for Health (HAS) and, for medicinal products not yet authorised under marketing authorisation, to the French National Agency for the Safety of Medicines and Health Products (ANSM).
These authorisations may apply to :
– a medicinal product prior to obtaining marketing authorisation for the indication in question (Pre-marketing authorisation AAP = AP1),
– a medicinal product which already has a marketing authorisation for the indication in question, prior to it being covered by the general health insurance system (Post-marketing authorisation AAP = AP2).
Interestingly, the product may or may not have marketing authorisation for another indication.
As indicated in the HAS doctrine, the granting of an AAP is reserved for specific medicinal products that meet the following 5 cumulative eligibility criteria:
- Efficacy and safety are strongly presumed in the indication in question
- The disease to be treated is serious, rare or disabling
- There is no such thing as “appropriate treatment”
- The treatment cannot be deferred
- The drug is presumed to be innovative.
The authorities examine all these criteria separately, and quite strictly.
This system also requires concrete commitments from the laboratories, which should not be underestimated and which need to be weighed up with the parent company.
- REGULATORY: The pharmaceutical company must undertake to submit a marketing authorisation application within 2 years for an AP1 or a registration application within one month of obtaining the marketing authorisation for an AP2. The timing of the application is therefore crucial to the project.
- LOGISTICS: The pharmaceutical company makes the product available within 2 months of the granting of the AAP and ensures that it can supply the product to allow continuity of treatment for patients initiated throughout the AAP. At the end of the AP, the exploitant pharmaceutical company ensures the continuity of the treatments initiated for a minimum period of one year, of which 3 months are covered by health insurance.
- FINANCIAL: The pharmaceutical company sets up a PUT-RD for data collection and the transmission of periodic summary reports. It funds the data collection withing the framework of an agreement signed with health establishments.
- The pharmaceutical company is also required to support prescribers in entering and monitoring the collection of real-life monitoring data for the drug, by providing them with the necessary resources.
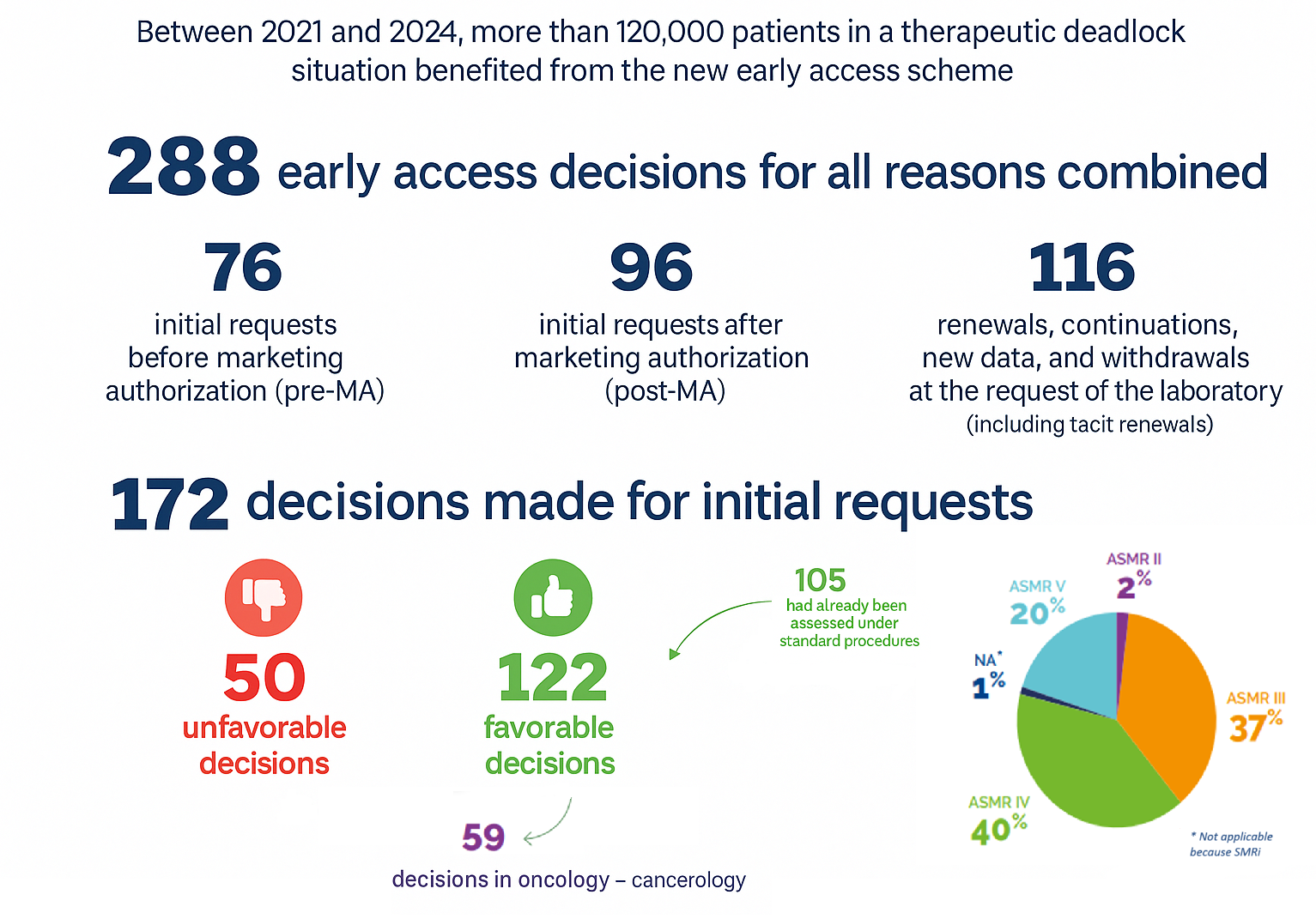
Since the implementation of the AP scheme in July 2021, the HAS has published a positive report covering three years of application :
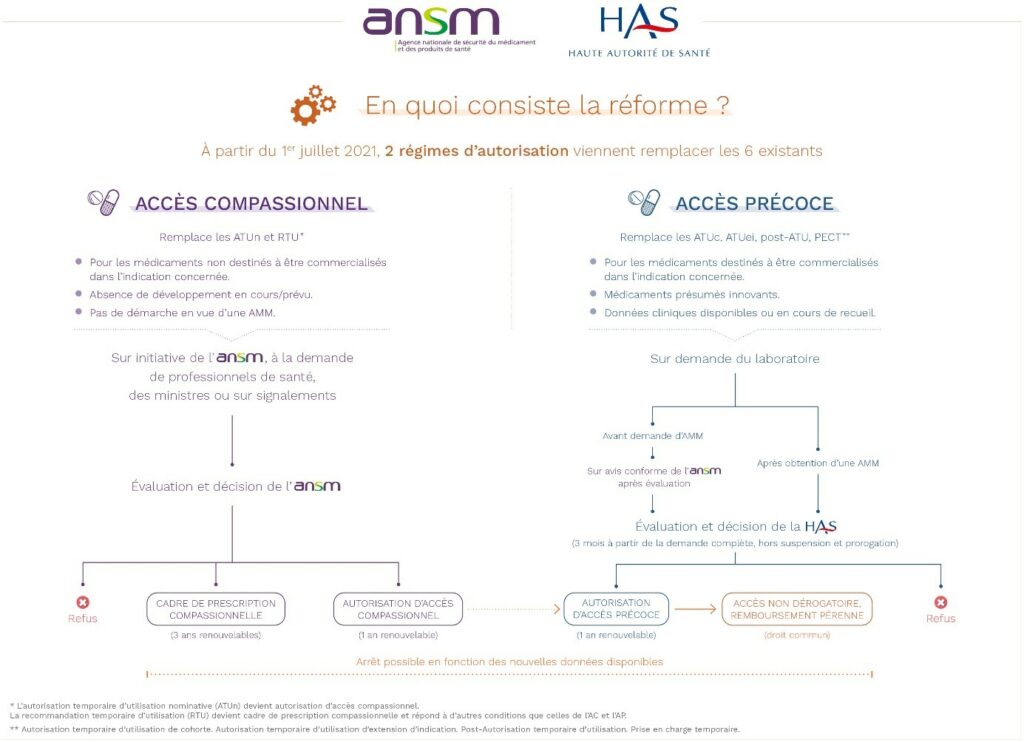
- Two types of compassionate access
This system covers two distinct cases, which have in common the fact that they concern a medicinal product used to treat patients suffering from illnesses for which there is no appropriate treatment, in a given therapeutic indication, without it being intended to obtain marketing authorisation in France. Applications are managed solely by the ANSM.
- 1st mechanism: this compassionate access is requested for an unauthorised drug not available in France by a hospital prescriber for a named patient, provided that the ANSM is able to presume a favourable benefit/risk ratio for a serious, rare or disabling disease: this is an individual and nominative compassionate access authorisation (AAC).
- 2nd mechanism: it is a framework for a practice, at the initiative of the ANSM, with a view to securing the practice of off-label prescribing of a medicinal product available in France, which has marketing authorisation for other indications, when it is the subject of well-established off-label prescribing on French territory : this is a compassionate prescribing framework (CPC).
Exceptions to compassionate access have been made in the following cases:
- Allowing nominative access to medicines in development for the indication: this is a “very early” compassionate access.
- The LFSS for 2024 also provides for the possibility of granting compassionate access authorisations in the event of a refusal for early access on the grounds that the drug is not considered innovative enough.
There are a number of eligibility conditions attached to the ANSM grant, which bring this scheme closer to early access and can be the gateway to it:
- treatment cannot be postponed;
- the patient cannot take part in any ongoing research;
- the company responsible for exploiting the medicinal product must undertake to submit an application for early access program within 12 months of the first ‘compassionate pre-approval’ (18 months for rare diseases).
Compassionate access schemes differ from early access programs in that their initiation does not rest with the manufacturer, who may be required to implement and fund a PUT-SP.
Thus, the reform has brought greater predictability for manufacturers and continuity of access up to standard reimbursement. In return, pharmaceutical companies are bound by a number of commitments.
According to the ANSM report, published in 2024, the use of compassionate access has been stabilising since the 2021 reform. In 2023, a relative decrease of 10% in compassionate access requests was observed. This decrease is partly linked to the granting of marketing authorizations for several Covid-19-related products, which had previously been the subject of numerous compassionate access requests.
Moreover, the number of medicinal products available under this scheme has remained stable, with 373 made available in 2023.
For these products, it may be necessary to appoint a pharmaceutical company to operate the medicinal product, in order to ensure import/distribution, pharmacovigilance, quality claims or medical information, as appropriate.
Pharmaceutical companies now have several years’ experience of these new mecanisms, and the trends that are emerging show a willingness on the part of the authorities to make innovative medicines available to French patients and to respond to the personal situations of patients who have reached a therapeutic impasse.
Article written by Lamya SAOUSSEN, Junior Regulatory Affairs and External Communication Advisor