How to proceed with non-pharmacopoeial excipients in the formulation of a medicinal product for human use?
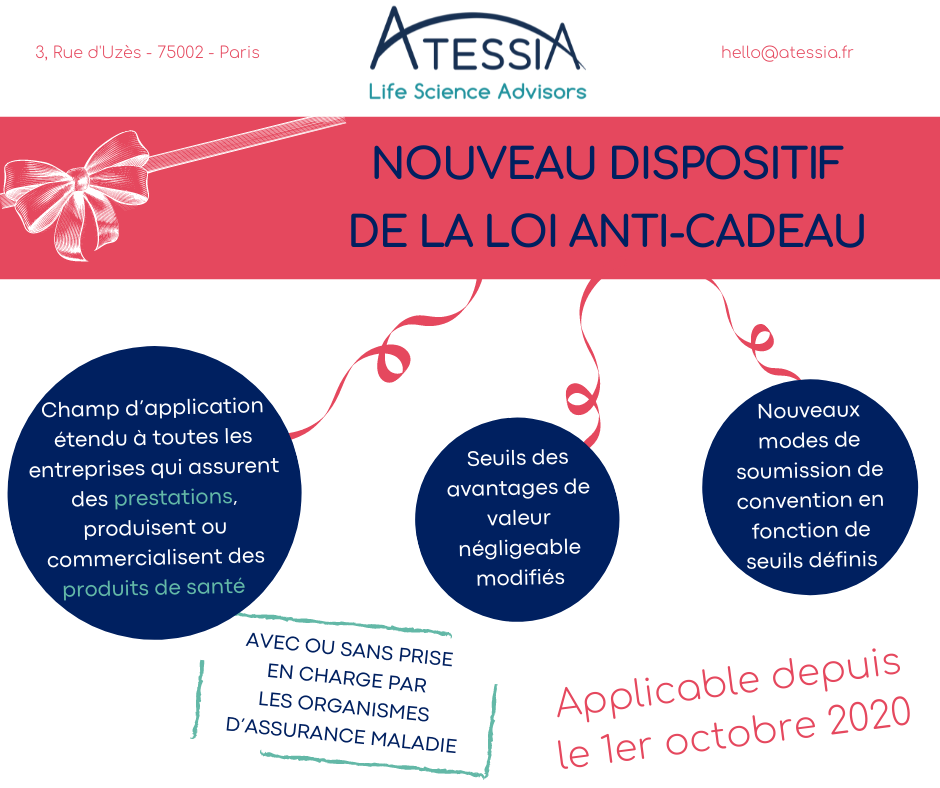
| French always do it different In many domains, French people like to stand out, either in a positive or negative way. In the Healthcare field, France has set a system that is highly effective, and very protective for the patient, but at the price of a heavy state involvement and of one of the most complex regulations. As a result, understanding the regulatory specificities of France is key for entering the French market. ATESSIA can help you in this process and provide you assistance and expertise. Here are a few areas where France follows its own, often complicated and restrictive, rules. |
– Excipients – inherently inactive – have a wide range of applications and are essential components in the formulation of medicines. However, few of these components are manufactured exclusively for pharmaceutical use. Often, the pharmaceutical use of an excipient represents only a minor fraction of its other industrial uses (food, cosmetics or others). However, the quality of excipients is crucial to ensure the quality, safety and efficacy of medicines.
– There is no registration or authorisation procedure prior to its use in a medicinal product. Implicit agreement for the acceptability of an excipient can only be obtained on the basis of a complete marketing authorisation dossier. Excipients used in the formulation of medicinal products can be classified into two main categories: known excipients (i.e. described in the literature, e.g. Handbook of Pharmaceutical Excipients, in the regulations, e.g. (EU) Regulation n°231/2003, or previously screened for risk/safety by an international organisation, e.g. JECFA, FAO, WHO) and unknown excipients, so-called “new excipients” (i.e. used for the first time in a medicinal product or via a new route of administration according to ICH M4Q).
– Among the known standard excipients used, the European guidelines of the EMA provide for two categories: those described in a pharmacopoeia (e.g. European, American, Japanese, French, British, etc.), and those not described in a pharmacopoeia. The latter are then subject to “in-house” control specifications, generally based on the excipient manufacturer’s own specifications, which may be consolidated by the MA holder depending on the use of the excipient in its formulation. It should be noted that in the case of mixed and co-processed excipients, a combination of one or more excipients described or not described in a pharmacopoeia may be considered.
– In Europe, the European Pharmacopoeia is the binding reference for substances for pharmaceutical use. Nearly 200 monographs for excipients are included. Increasingly, they include additional pharmaco technical specifications depending on the use of the excipient. The aim of the European Pharmacopoeia excipient monographs is to provide authorities and patients with a guarantee of acceptable quality. The national pharmacopoeias of the EU Member States are also considered as reference documents that can be used without providing a specific justification in the marketing authorisation dossier. A reference to the “current edition” of the European/national pharmacopoeia in the marketing authorisation dossier is expected to avoid subsequent maintenance changes to the marketing authorisation dossier.
– For the same excipient, depending on its various possible origins and uses, different additional requirements may also be necessary: e.g. with regard to a chemical risk (residual solvents according to ICH Q3C, elemental impurities according to ICH Q3D, nitrosamine impurities, etc.), with regard to a bacteriological risk (mycotoxins, endotoxins, sterility, etc.), or with regard to a viral risk (TSE/BSE).
– Although also accepted in Europe under certain conditions, references to monographs described in other pharmacopoeias (i.e. outside Europe and considered as third country or unofficial pharmacopoeias) must be duly justified in the marketing authorisation dossier. In this case, a copy of the monograph accompanied, if necessary, by the validation of the analytical procedures contained in this monograph and, if necessary, a translation should be provided in the marketing authorisation dossier. Updates to these monographs should be declared via the submission of MA variations to the authorities.
– In cases where the excipient used in the formulation is known but has no control monograph in a pharmacopoeia (e.g. surfactant for a skin solution), specifications should be set by the supplier to ensure that the quality of the raw material is maintained on a continuous basis, and reflects both the inherent properties of the excipient and its manufacturing process. These specifications are usually taken over by the user of the excipient and consolidated where appropriate. Consideration of the following parameters is recommended in establishing these internal control specifications: physical characteristics, identification tests, purity tests, assay and other relevant tests that may influence the performance of the pharmaceutical form. In addition, it should again be demonstrated that the methods used provide accurate, reproducible and repeatable results for the characteristic tested and are therefore validated.
– It should be remembered that it is the responsibility of the drug product manufacturer to ensure the quality of all raw materials used in the manufacture of a medicine. By regularly auditing the excipients producer, the end user is able to determine whether adequate controls are in place to ensure that the producer is capable of producing a product of appropriate quality. It should be noted that several guidelines and practical guides are available to manufacturers and users of pharmaceutical excipients from IPEC (The International Pharmaceutical Excipients Council) on the following topics: excipient compositional profiling, excipient qualification, excipient safety, development of certificates of analysis, determination and auditing of Good Manufacturing Practices (GMP) for manufacturers, development of technical agreements/specifications, etc.
– Finally, since 2015, the European regulation imposes on excipients manufacturers the obligation to comply with the appropriate GMP determined by the drug product manufacturer on the basis of a formalised risk assessment (reference document to be provided in case of inspection). An ANSM doctrine, currently being finalised, will soon explain the inspection procedures for excipient manufacturers with regard to the provisions of Article L.5138-3 of the Public Health Code.
ATESSIA assists you in the drafting of your MA and variation files in the context of your activities related to all types of pharmaceutical excipients.
Article written by Isabelle MOUVAULT, Pharmaceutical Affairs Advisor