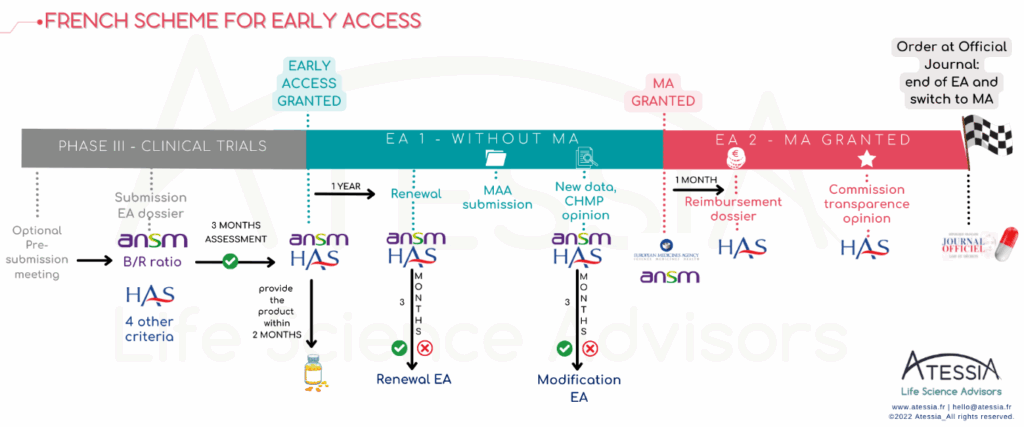
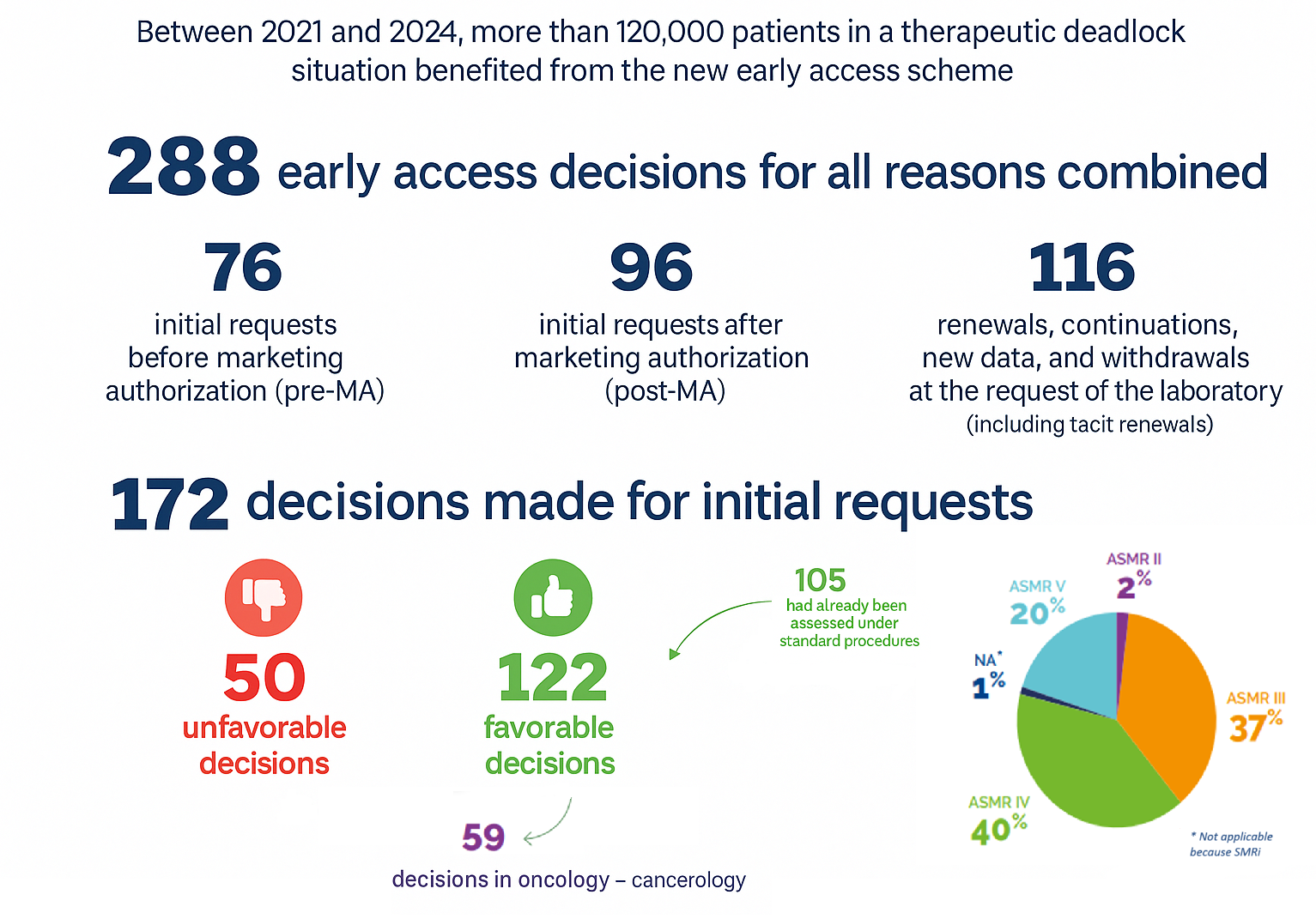
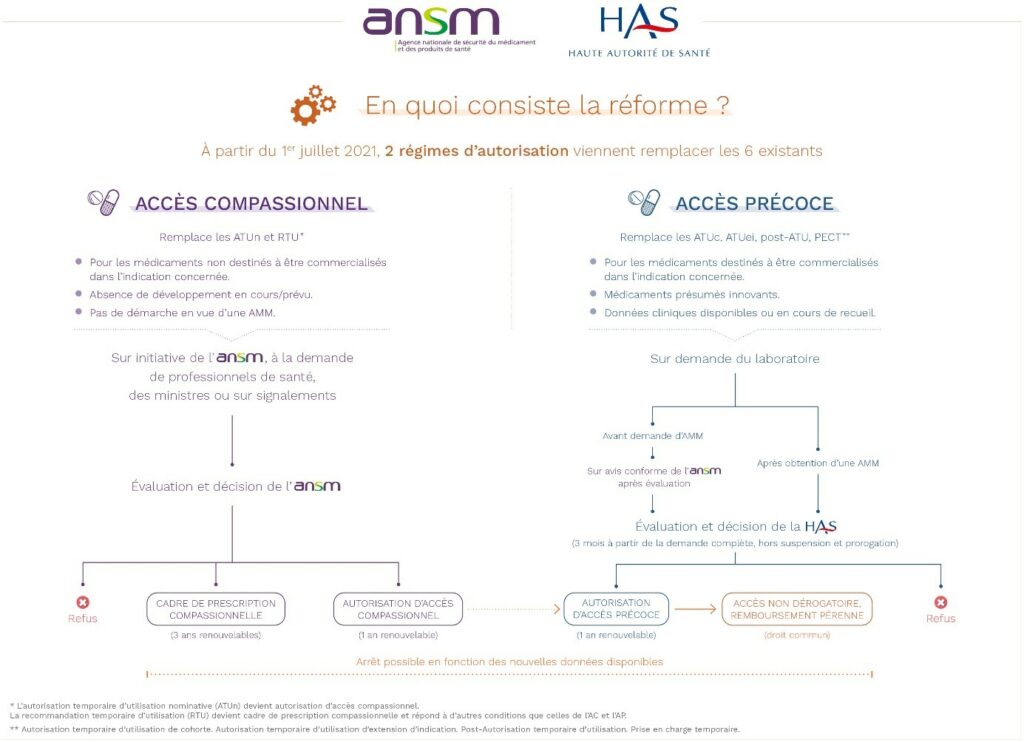
CEP : what’s that?
In Europe, three distinct possibilities exist for presenting information relating to the active substance, from a qualified manufacturer, used in a medicinal product in the marketing authorisation file (MA file):
– present a complete documentation (sections 3.2.S.1 to 3.2.S.7.3 100% completed according to the requirements of NtA-Vol 2B);
– present an Active Substance Master File (“ASMF“) which contains an open (non-confidential) part and a closed (confidential) part;
– present a Certificate of Suitability to the monographs of the European Pharmacopoeia (CEP).
In this article we will focus on defining what the CEP consists in and why this possibility for recording a source of active substance is particularly interesting.
The CEP is a document issued by the European Directorate for the Quality of Medicines and Healthcare (“EDQM“) following an official European procedure for Certification of suitability to the monographs of the European Pharmacopoeia (Ph. Eur.) (known as the “Certification procedure”).
This document certifies that the quality of the substance in question can be adequately controlled by the corresponding Ph. Eur. monograph(s), supplemented if necessary by additional tests appearing on the CEP. It is thus based on the evaluation by EDQM experts of a file submitted by the applicant (in the same way as by the experts of the competent authorities for the MA application file).
However, a CEP does not allow to confirm compliance with Good Manufacturing Practices (GMP) of the substance concerned and cannot replace the GMP certificate. A CEP can therefore be issued by the EDQM with or without inspection of the manufacturing site. Also a CEP does not replace a certificate of analysis and does not guarantee that a batch of the substance is of appropriate quality. Therefore, vigilance must remain required by the future user of the substance, with the implementation of controls upon receipt of the substance.
The Certification procedure is applicable to substances for which a general or specific monograph has been adopted by the European Pharmacopoeia Commission (e.g. substance of synthetic origin [active substance or excipient], herbal drugs, herbal drugs preparations, etc.). This procedure does not apply to direct products of genetic expression (proteins), nor to products obtained from human tissues, nor to vaccines or products and preparations derived from blood. Furthermore, although this may have been the case in the past, the EDQM does not accept new CEP applications for substances of biological origin.
A public list of CEPs with their status is available without access restriction in the EDQM certification database.
Details of the content of the CEP application file are provided on the EDQM website. New requirements are now in force for the constitution of files by applicants with the entry into force of CEP 2.0 since September 1, 2023.
- The different categories of CEP
There are currently several types of CEPs issued by EDQM depending on the evaluation carried out, including the following simple CEPs:
– Certificate of chemical purity and microbiological quality (“Chemical CEP”) à e.g.: paracetamol
– Certificate for herbal drugs and herbal drugs preparations (“Herbal CEP”)à e.g.: lavender oil
– TSE certificate for substances of animal origin potentially subject to transmissible spongiform encephalopathy (“TSE CEP”) à e.g.: gelatin
Combined CEPs may also be granted, as follows:
– Certificate of chemical purity/microbiological quality and sterility à e.g.: sterile amoxicillin sodium
– Double certificate (chemical + TSE) à e.g.: cholecalciferol
– Double certificate (chemical + TSE) also covering sterility
It is possible to claim a particular quality for a substance, this must be duly demonstrated in the file (e.g. sterile, micronised, crushed substance, etc.): this quality will be indicated in the subtitle on the CEP. Furthermore, it is possible to request a CEP for a particular polymorph (as a quality), even if the mention “the substance presents a polymorphism” does not appear in the “Characters” section of the corresponding specific Ph. Eur. monograph.
- Recognition of the CEP outside Europe
Although it is not mandatory for the marketing of substances, the Certification procedure constitutes the preferred option for ensuring that a substance entering in the composition of a medicinal product complies with the specifications of the European Pharmacopoeia, as well as for demonstrating the compliance with TSE risk requirements.
From a regulatory perspective, CEPs are accepted in all EU Member States and in States that have signed the Convention relative to the elaboration of a European Pharmacopoeia (including the United Kingdom), with the exception of Ukraine.
According to the information received by the EDQM, the following countries accept CEPs, sometimes under conditions (non-exhaustive list): South Africa, Albania, Algeria, Saudi Arabia, Australia, Azerbaijan, Canada, Georgia, Ghana, Israel, Kyrgyzstan, Malaysia, Morocco, Moldova, New Zealand, Uzbekistan, Singapore, and Tunisia. CEPs are also accepted (under conditions) by the Taiwan Food and Drug Administration.
CEPs may therefore be accepted in other countries (non-EU or Ph. Eur. members), at the discretion of the authorities of these countries. In such cases, the Competent Authorities will decide on the scope and conditions of acceptance of the CEP (e.g. submission of a partial or complete ASMF in addition to the CEP). The EDQM indicates that it is therefore important to check, in advance, the acceptability and conditions associated with the use of a CEP in these countries.
Additional requirements may be applied by some non-EU states (e.g. signed, dated and version-controlled documents for specifications and analytical procedures).
In terms of the MA file, section 3.2.S of the MA holder/applicant for the active substance should also be adapted by making the necessary references to the CEP and/or by providing the data specific to the manufacturer(s) of the finished product using the substance covered by a CEP in the relevant sections of the CTD.
As a conclusion, the CEP is an essential document for marketing authorisation files in Europe and its evaluation by the EDQM encourages preferred use, the updating of which is less constraining in regulatory terms than other options (e.g. ASMF). Although recognised by a large majority of countries worldwide, it may not be sufficient in itself and may have to be subject to specific requirements of the country in which the MA is registered.
Source: EDQM -FAQs (February 2024)
Article written by Isabelle MOUVAULT, Pharmaceutical Affairs Senior Consultant