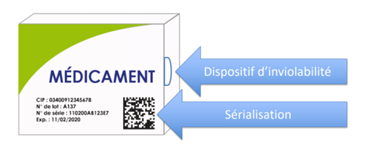
What Are the French Rules for Influencers on Social Networks?
Law No. 2023-451 dated June 9, 2023, which aims to regulate commercial influence and combat abuses by influencers on social media platforms, was amended in November 2024 to align with the European legal framework. As a reminder, this law defines the concept of an “influencer”.
What is an Influencer?
Influencers are any “(…) natural or legal persons who, for a fee, mobilize their reputation among their audience to disseminate content to the public via electronic means. Their goal is to promote either directly or indirectly, goods, services or any cause. They engage in the activity of commercial influence through electronic means” (Article 1).
Examples include a patient, a healthcare professional or a person with a strong reputation.
What are the Law’s Obligations?
Transparency obligation:
Influencers must disclose the commercial intent behind their content:
- When it is not already evident from the context,
- Through a clear, legible, and understandable label such as “Advertisement” or “Commercial Collaboration,” or an equivalent adapted to the activity and media format.
Unlike the previous version of the law, it is no longer mandatory for the label to appear “on the image or video, in all formats, throughout the entire promotion.”
Supervision of Published Visuals:
- Images should be labelled as “Retouched Images” if they undergo processing to slim or thicken a silhouette or modify face appearance.;
- Images should be labelled as “Virtual images” if any artificial intelligence process has been used to generate or modify a face or silhouette.
Supervision of Dropshipping Activities:
Influencers are obliged to provide the buyer with all pre-contractual information related to a distance sales agreement. This includes the identity of the supplier and confirmation of product availability. Failure to provide this information can result in influencers being held accountable.
What is Prohibited?
Direct or indirect promotion of the following products and services is prohibited:
- Aesthetic procedures, processes, techniques, and methods referred to in Article L. 1151-2 of the French Public Health Code, as well as interventions referred to in Article L. 6322-1 of the same code (including aesthetic medical devices (DMs) listed in Annex XVI of Regulation 2017/745 MDR);
- Procedures, processes, techniques, and methods presented as comparable to, preferable over or substitutes for therapeutic procedures, protocols, or prescriptions;
- Products considered as nicotine-based that can be consumed and are made, even partially, of nicotine.
What are the Penalties?
Violators may face a fine of up to 300,000 euros and a prison sentence of up to 2 years.
To ensure consumer protection, a dedicated team has been set up within the DGCCRF (a French authority, Direction Générale de la Concurrence, de la Consommation et de la Répression des Fraudes), and reports can be submitted via the Signal conso website.
Existing sanctions are reinforced and graduated. The following acts are punishable:
- Failure to indicate the advertising nature of a video or photo posted by an influencer is now considered a misleading commercial practice;
- Promotion of a prohibited or regulated product carries the same penalties as online advertising;
Additionally, the authorities have been granted a new power of injunction with penalties. This allows them to compel an influencer to remove non-compliant content or for platforms to suspend the influencer’s account promptly.
Judges and supervisory authorities will tailor penalties according to the severity of the act.
What About Drugs and Medical Devices?
The promotion of medicines to the general public is regulated by the French Public Health Code.
Promotion to the general public of a medicine, MD, or IVDMD cannot refer to recommendations from:
- Scientists or healthcare professionals,
- Individuals who, although not scientists or healthcare professionals, may encourage product use due to their notoriety—unless the advertisement concerns a Class I or IIa medical device.
Need assistance in managing influencer communication under contract with your laboratory? Our expert consultants are available to discuss your concerns.
Article written by Marie–Amélie Marcq, Regulatory and Pharmaceutical Affairs Senior Consultant