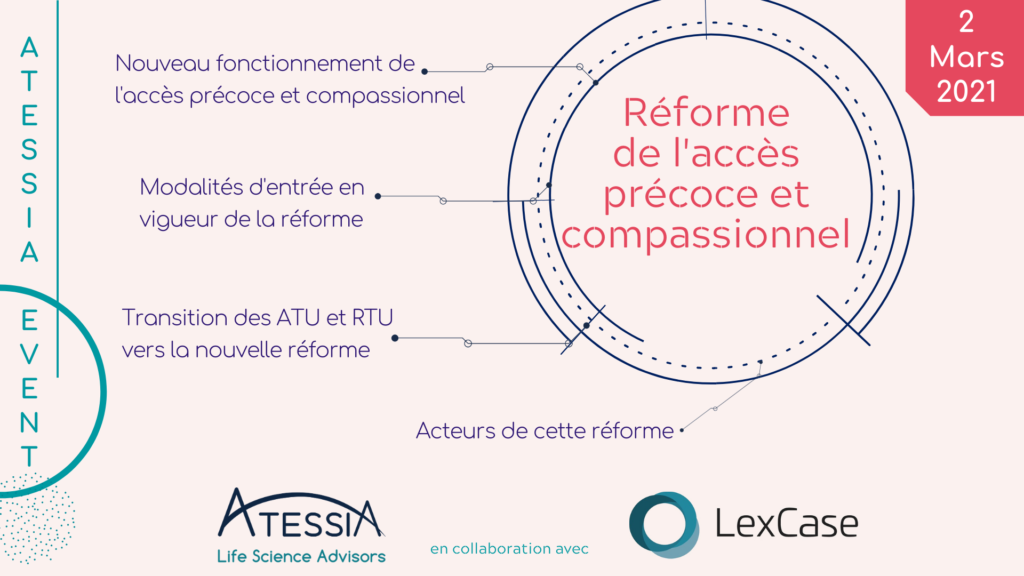
Atessia Event – Early and Compassionate Access Reform
Atessia in collaboration with the law firm LexCase conducted a training course about “Early Access Reform”. On the agenda of this presentation :
Reminder of the existing framework
Decryption of the completely redesigned device
Implications and prospects
More than 100 people participated in this event!