Presentation of ICH (roles, history, missions, etc.)
ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) is an organisation that brings together regulatory authorities and the pharmaceutical industry to discuss scientific and technical aspects of drug registration.
In particular, this instance develop harmonised guidelines for global pharmaceutical development: the “ICH guidelines“.
Since its founding in 1990, the ICH’s mission is to promote and support harmonisation worldwide to ensure that safe, effective and high-quality medicines are developed and registered efficiently.
Like other organisations, ICH has had to adapt to an increasingly globalised drug development industry.
ICH was incepted in 1990 and was formerly named the International Conference on Harmonisation (ICH). At the first ICH Steering Committee meeting of ICH, it was decided that the topics selected for harmonisation would be divided into three distinct domains in order to reflect the criteria which are the basis for approving and authorising new medicinal products: Safety (S), Quality (Q) and Efficacy (E).
Cross-cutting topics that do not fit uniquely into one of these three categories fall into the Multidisciplinary (M) domain. These include the “MedDRA” medical terminology, the “CTD” common technical format and the development of “ESTRI” electronic standards for the transfer of regulatory information.
The ICH Assembly brings together all members and observers of the ICH Association as the overarching governing body of the organisation.
Since October 2015, ICH has grown as an organisation and now includes 23 Members and 38 Observers.
As an observer, the EDQM contributes to the development of ICH guidelines in a number of relevant areas (e.g.: control of impurities, development and validation of analytical procedures and continuous manufacturing).
The evolutions of ICH
Since its creation, the ICH has gradually evolved to meet the increasingly global challenges of the pharmaceutical industry.
The key dates are listed below:
>1980s: Lauch of harmonisation of regulatory requirements by the EC as Europe moved towards the development of a single market for pharmaceuticals. At the same time, discussions occur between Europe, Japan and the US on possibilities for harmonisation.
>1989: Specific plans for action for harmonisation begin to materialise at the WHO Conference of Drug Regulatory Authorities (ICDRA) in Paris. Soon afterwards, the authorities approached International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) to discuss a joint regulatory-industry initiative on international harmonisation, and ICH was conceived.
>April 1990: Birth of ICH at a meeting hosted by EFPIA in Brussels. Representatives of the regulatory agencies and industry associations of Europe, Japan and the US met, primarily, to plan an International Conference but the meeting also discussed the wider implications and terms of reference of ICH.
Now in its fourth decade of activity, ICH’s attention is directed towards extending the benefits of harmonisation beyond its founding regions (Europe, USA and Japan). In 2015, to facilitate this, a series of organisational changes took place. These changes constituted a number of reforms including: increasing international outreach; changing ICH’s governance structure; disseminating more information on ICH processes to a wider number of stakeholders; and establishing ICH as a legal entity to provide for a more stable operating structure.
The resulting ICH association establishes an Assembly as the over-arching governing body with the aim of focusing global pharmaceutical regulatory harmonisation work in one venue that allows pharmaceutical regulatory authorities and notably concerned industry organisations to be more actively involved in ICH’s harmonisation work.
The ICH’s work products
The work carried out by the ICH falls into four areas: harmonisation activities, the development of guidelines, the development of standards (MedDRA dictionary, CTD format or ESTRI electronic standards) and other work (e.g.: the development of discussion papers).
The ICH guidelines development process
ICH harmonisation activities fall into 4 categories: Formal ICH Procedure, Q&A Procedure, Revision Procedure and Maintenance Procedure, depending on the activity to be undertaken.
Each harmonisation activity is initiated by a Concept Paper which is a short summary of the proposal. Depending on the category of harmonisation activity a Business Plan may also be required. The Business Plan outlines the costs and benefits of harmonising the topic proposed by the Concept Paper.
The Formal ICH Procedure (a step-wise procedure consisting of 5 steps) is followed for the harmonisation of all new ICH topics.
The procedure is initiated with the endorsement by the ICH Assembly of a Concept Paper and Business Plan. An Expert Working Group (EWG) is subsequently established.
The EWG works to develop a draft Guideline and bring it through the various steps of the procedure which culminate in Step 5 and the implementation in the ICH regions of a Harmonised Guideline.
The five steps are as follows:
- Step 1: Consensus building
- Step 2a: Confirmation of consensus on the Technical Document
- Step 2b: Adoption of draft Guideline by Regulatory Members
- Step 3: Regulatory consultation and Discussion
- Step 4: Adoption of an ICH Harmonised Guideline
- Step 5: Implementation
The ICH guidelines implementation process
At Step 5 of the ICH process, harmonised ICH Guidelines are implemented by ICH Regulatory Members and Observers within their respective country/region. This is in line with the ICH Articles of Association and the aim and intention that all ICH Regulatory Members should implement all ICH Guidelines.
For ICH Regulatory Observers, implementation of (certain) ICH Guidelines is a pre-requisite to become an ICH Regulatory Member.
ICH Guidelines are implemented in accordance with the applicable national/local/regional rules, with the stage of implementation of all ICH Guidelines also being dependent on when a Member or Observer has joined ICH.
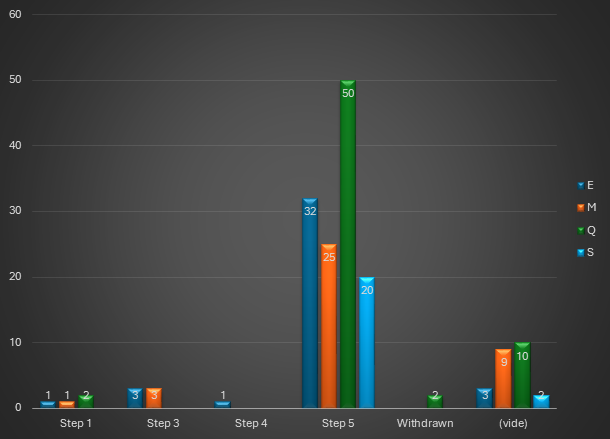
In April 2025, a total of 163 guidelines (all domains combined) were published on the ICH website, broken down as shown in the graph below:
Future ICH work impacting global regulation
ICH continues to propose guidelines on pharmaceutical development topics requiring regulatory harmonization, or where a lack of recommendations has been identified. This is the case, for example, of the (long-awaited) draft ICH Q3E guideline on extractables and leachables.
In addition, the updating of current guidelines is also supported, in order to take account of scientific progress. Examples include the recent update of the ICH Q2 guideline on analytical validation, and the forthcoming revision of the ICH Q1A-Q1F series of stability guidelines.
Sources:
EDQM – Pharmacopoeial Harmonisation
Article written by Isabelle MOUVAULT, Pharmaceutical Affairs Senior Consultant

